Depression has an immense impact on individuals and their families. Depression takes a toll on everyone and affects each individual differently, but the costs can be severe. It effects more than just the pocketbook. It is more than lost hours at work or possible unemployment or even the high expenses of medication treatment and coping with medication side effects. It is the severe costs that symptoms of depression take daily from the countless individuals who suffer, as well as from their loved ones. It is the impact that depression has on you and your relationships. It is the impact on your work, your leisure, and your way of life.
Most people with Major Depressive Disorder (MDD) are able to find relief from their depression symptoms through counseling (psychotherapy) and/or medication. However, if you've been treated for depression but your symptoms haven't improved, you may have treatment resistant depression (TRD). The FDA defines treatment resistant depression as a "failure of at least two antidepressant trials, given at adequate doses, for 6–8 weeks while ensuring adequate treatment adherence, during a MDD episode".
If you are fighting a personal battle with depression and haven’t achieved success with traditional medication treatment, you are not alone. In fact, over 5 million adults in the United States are treated for depression and unable to find relief with antidepressant medication, while others may experience improvement but find the side effects of the medication intolerable.
Treatment Resistant Depression Treatments
High Country Behavioral Health now offers two alternatives for treating Treatment Resistant Depression: Transcranial Magnetic Stimulation (TMS) and Spravato (esketamine) Treatment.
Magstim TMS Therapy in our Idaho Falls office and Neurostar TMS Therapy in our Evanston WY office.
Spravato (esketamine) is available at our Idaho Falls and Pocatello, Idaho locations, as well as our Evanston and Douglas, Wyoming locations.
Transcranial Magnetic Stimulation (TMS)
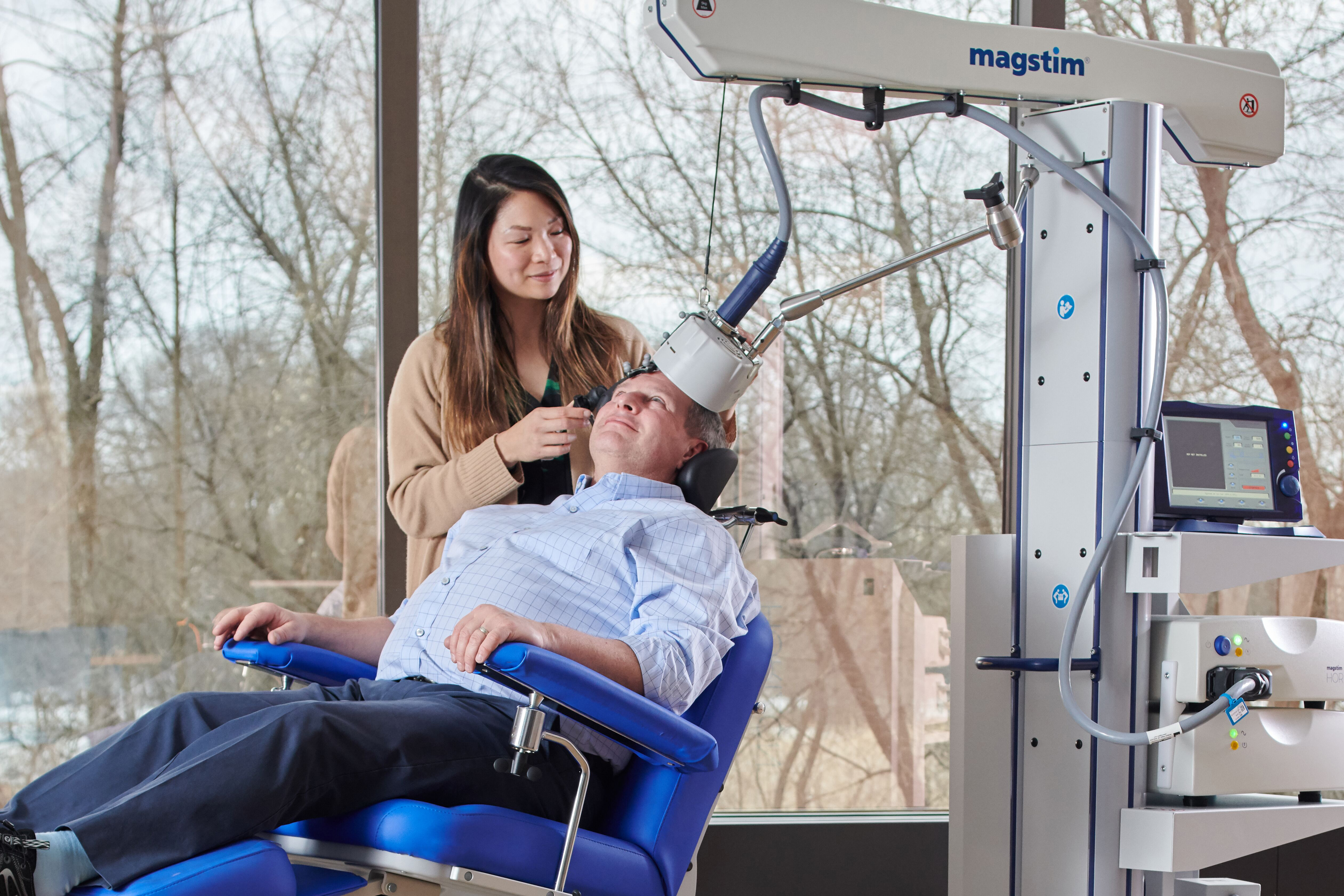
Transcranial Magnetic Stimulation (TMS) is a form of safe, non-invasive brain stimulation. Its application has been used for neuroscientific and clinical research around the world for over 30 years. The US Food and Drug Administration (FDA) cleared Magstim TMS Therapy systems for the treatment of Major Depressive Disorder in adult patients who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode. It continues to be recommended by the American Psychiatric Association (APA) for Major depressive Disorder (MDD) since 2010.
Magstim TMS Therapy uses short pulses of magnetic energy to stimulate nerve cells in the brain. These magnetic pulses are delivered to the area in the brain that researchers believe is responsible for emotional judgement and mood regulation. The rapid magnetic pulses created by the TMS system, pass through the skull and generate an electric current in the brain directly under the treatment coil. These electric currents cause neurons to fire and stimulate surrounding brain cells.
When a nerve cell ‘fires’, an electrical impulse travels along its length. It communicates with other nerve cells by releasing neuro-transmitters, which create an electrical impulse in other cells. In depressed patients, the electrical activity in certain areas of the brain have been shown to be reduced.
TMS uses a focused electromagnetic coil, to rapidly pulse a magnetic field to the targeted area of the brain. The magnetic pulses induce an electrical current in the brain, stimulating the nerve cells, increasing the brain activity to normal levels.
High Country Behavioral Health offers Magstim TMS Therapy in our Idaho Falls office and Neurostar TMS Therapy in our Evanston WY office.
SPRAVATO® (esketamine)
SPRAVATO® is an FDA approved prescription nasal spray, used along with an oral antidepressant, to treat adults with treatment resistant depression.
SPRAVATO® works differently than other medications for treatment resistant depression. Today’s most commonly used oral antidepressants are thought to treat depression by increasing levels of neurotransmitters (serotonin, norepinephrine and dopamine) in areas of the brain that affect mood. SPRAVATO® works differently, in that, it targets the N-methyl-D-aspartate (NMDA) receptor. The exact way that SPRAVATO® works is unknown. However, it has shown positive results in clinical trials.
In a short-term clinical study of adults with treatment resistant depression, those who took SPRAVATO® and an oral antidepressant experienced a greater reduction of depression symptoms at four weeks, compared to those who received a placebo and an oral antidepressant. In a long-term study after 16 weeks of therapy, patients who stayed on SPRAVATO® were less likely to experience a return of depressive symptoms than those who stopped therapy.
SPRAVATO® is not right for everyone. Your medical provider will work with our team to determine if you are a potential candidate for SPRAVATO®.
Learn more about Spravato and its potential side effects.
Find out more in this SPRAVATO® video.